Lights and shadows of implantable cardioverter defibrillators implantations in hypertrophic, dilated and arrhythmogenic right ventricular cardiomyopathies.
Posted: April 29, 2013 Filed under: Medical Devices, Opinions | Tags: bradycardia, defibrillator, eHealthcare, Health 2.0, ICD, implantable cardioverter defibrillators, lights, Medical Devices, pacemaker, SCD, sudden cardiac death Leave a commentIn the past decades, implantable cardioverter defibrillators (ICDs) have definitively proved to be superior to antiarrhythmic drugs for the prevention of arrhythmic sudden cardiac death (SCD). Several studies have proved this hypothesis both for primary or secondary (i.e. after a sustained ventricular tachycardia with hemodynamic compromise or ventricular fibrillation) prevention of SCD. However, some complications often occur with ICD implantation, which may be particularly serious for young individuals.
Indications for ICD implantation in young people are mostly ion channel diseases and cardiomyopathies. The latter are structural abnormalities of the myocardium, related to genetic abnormalities, predisposing to malignant ventricular arrhythmias and requiring ICD for the prevention of SCD in some cases.
Hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is one of the most common cardiomyopathies and occurs in the 0.2% of the general population1. It is characterized by localized asymmetrical hypertrophy of the heart and disarray of myocardial cells and filaments. This leads to impaired transmission of electrophysiologic impulses, with an unstable electrical substrate, potentially causing lethal ventricular tachyarrhythmias and SCD.
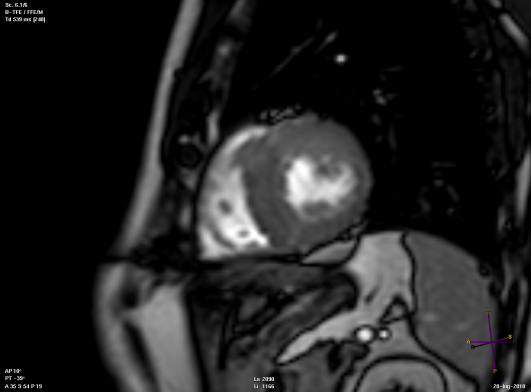
Short axis view of a cardiac magnetic resonance imaging of a patient affected by hypertrophic cardiomyopathy involving the interventricular septum and the anterior wall of the left ventricle.
ICD implantation in HCM: lights
A minority of patients with HCM are judged to be at increased risk for SCD, whose rate is estimated to be about 1% per year 2. However, malignant ventricular arrhythmias remains the most frequent cause of death in this population.
Beta-blockers have failed to demonstrate significant protection from SCD in HCM patients. Type I and III anti-arrhythmic drugs, including amiodarone, have been abandoned because of inefficacy and pro-arrhythmic side effects. Experiences with endocardial and epicardial mapping and ablation in HCM are poor and limited to highly selected patients.
ICDs, conversely, have been proved effective in terminating life-threatening ventricular tachyarrhythmias in HCM and are currently considered the only available tools altering the natural course of the disease and prolonging life.
Appropriate intervention rates of ICD in HCM have been esteemed to be about 11% and 4% for secondary and primary prevention respectively3.
Current recommendation for ICD in HCM
HCM has heterogeneous clinical manifestations in different individuals and a not well predictable clinical course. Targeting HCM-patients for prophylactic ICD implantation can therefore be challenging. However, some “risk factors” have been individuated, that commonly guide the decision for ICD implantation. Current international guidelines recommend ICD in all cases of secondary prevention or when familiar history of SCD, marked left ventricular (LV) hypertrophy or recent unexplained syncopal episodes are present. The role of ICD is uncertain for non-sustained VT or an abnormal blood pressure response with exercise.
ICD implantation in HCM: shadows
Previous studies have reported up to 5.1%/year rate of ICD-related complications in HCM4.
The most frequently reported is inappropriate shock delivery, mostly due to erroneous detection and treatment of atrial fibrillation by the ICD. A retrospective study on 334 consecutive HCM patients with an ICD, showed a rate of appropriate vs. inappropriate shock delivery of 2.3 and 4.6%/year5. Moreover, a recent meta-analysis involving 2190 ICD-recipients affected by HCM, showed a rate of appropriate vs. inappropriate ICD interventions of 3.3 vs. 4.8%/year respectively4.
Another extremely rare complication with ICDs in HCM, is represented by loss of capture due to high pacing thresholds (i.e. energy required for effective right ventricular pacing). This is related to ventricular hypertrophy and may be prevented by accurate individuation of the optimal pacing threshold during ICD programming.
Lengthened mitral valve leaflets, and left ventricular outflow tract obstruction due to LV hypertrophy, also confers a relatively higher risk of infection and endocarditis to HCM patients, which must be considered when an ICD implantation is programmed. Moreover, since ICD implantation in HCM is commonly performed at a relatively young age, patients are supposed to necessitate of a number of interventions for pulse generator substitution over their entire life. For this reason, risk of infection and endocarditis (related to ICD substitutions) is amplified. Eventual needing of ICD and intra-cardiac leads extraction over time must be considered. This is a high-risk procedure, possibly complicated by cardiac tamponade, shock, anemia, arrhythmias or even death.
Finally, ICD implantation can be accompanied by depression, anxiety, reduced quality of life, particularly in young people.
Idiopathic dilated cardiomyopathy
Idiopathic dilated cardiomyopathy (IDCM) is a myocardial disease characterized by LV dilation and systolic dysfunction, commonly resulting in heart failure (HF) and for whom an etiological basis cannot be identified. IDCM is relatively rare (36.5% new cases/year/100.000 persons) but accounts for nearly 10.000 deaths/year in the United States, both due to HF and arrhythmic SCD6.
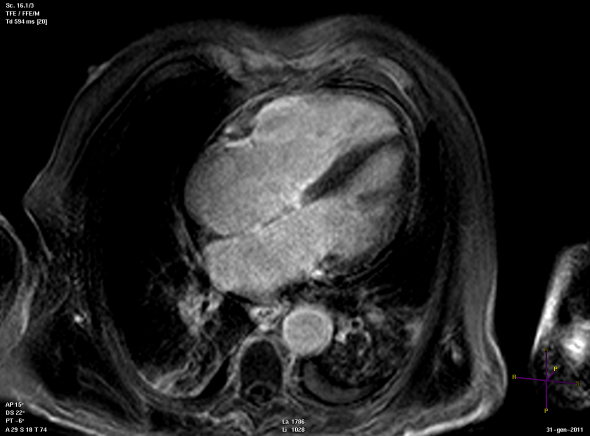
Long axis view of a cardiac magnetic resonance imaging of a patient affected by dilated cardiomyopathy
ICD implantation in IDCM: lights
ICD are effective for the prevention of SCD in IDCM and can favorably alter the natural course of the disease. Previous studies have showed a rate of appropriate interventions of 5 to 7.5%/year in IDCM-patients with an ICD implanted for primary prevention 7-8. A large meta-analysis has showed a reduction of mortality with ICD of about 3.5%/year compared to the best medical therapy in IDCM 9.
Recommendation for ICD in IDCM
The most important risk factor for ventricular arrhythmias in primary prevention of SCD in IDCM, is represented by severe contractile dysfunction, quantified by the measurement of the ejection fraction (EF) at echocardiography. Functional status, assessed by the New York Heart Association (NYHA) class, also plays a role.
Current international guidelines recommend ICD always for secondary prevention. In case of primary prevention, ICD must be implanted in patients with LVEF ≤35% and NYHA class II and III10. However, in a real-life setting, the decision making for patients with IDCM is more complex, because issues such as additional cardiac resynchronization therapy (CRT), co-morbidities, the potential to improve LVEF over time, and eventual genetic etiology also should be considered. With regard to the last issue, carriers of mutations in gene encoding for laminin, are tough to be at increased risk of complete atrio-ventricular block, malignant ventricular arrhythmias and SCD, and may therefore require ICD implantation independently from the EF%.
ICD implantation in IDCM: shadows and warnings
Some shadows obscure the scenario of SCD prevention with ICDs in IDCM.
An important issue regards the high number of patients to be treated in order to save one life because of a currently adopted risk stratification process that appears to lack specificity.
Another topic is the correct timing for ICD implantation for primary prevention. A post hoc analysis of the DEFINITE trials 11 showed that only patients who had received their ICD not later than 3 months after the diagnosis of IDCM would have benefit from implant. However, a significant number of IDCM-individuals show marked improvement of the EF over time, up to values higher than those for which ICD implantation is currently recommended. The goal therefore, appears to be an early and correct individuation of those subjects who will have a negative clinical course, and will therefore require an early ICD implantation for primary prevention.
Similarly to HCM, ICD-related complications have been also reported by various studies, among whom:
- inappropriate shocks, with consequent reduction of quality of life;
- infections;
- risks related to eventual lead extractions;
- depression and anxiety.
Arrhythmogenic right ventricular cardiomyopathy
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a genetic cardiomyopathy characterized by progressive fibro-fatty replacement of the right ventricular myocardium, sometimes extending also to the left ventricle. The clinical presentation is usually related to ventricular tachycardia (VT) with a left bundle branch block pattern or ventricular fibrillation (VF) leading to SCD. ARVC is a progressive disease ultimately leading to HF.
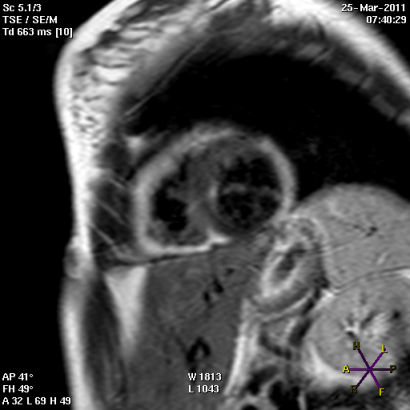
Panel A
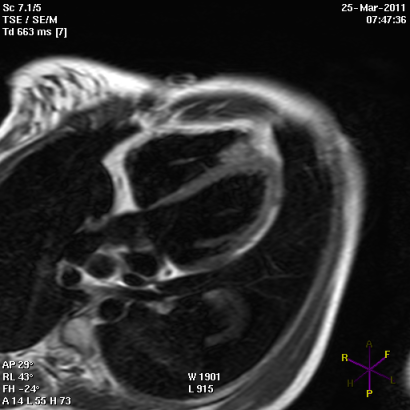
Panel B
Short (Panel A) and long axis views (panel B) of a cardiac magnetic resonance imaging of a patient affected by arrhythmogenic right ventricular cardiomyopathy, with evident fibro-fatty replacement of the right ventricle wall, the apex and partial involvement of the left ventricle wall.
ICD implantation in ARVC: lights
The therapeutic options for ventricular arrhythmias in ARVC include catheter ablation and antiarrhythmic drugs such as beta blockers, sotalol and amiodarone. However, these strategies have proved to improve symptoms but not to increase survival. Nowadays, ICD remains the most effective safe-guard against SCD in ARVC.
In a large multicenter international study enrolling ARVC patients with an ICD implanted for primary prevention, appropriate device interventions were observed in one fourth of patients after 5 years. The annual rate of potentially “life-saving” shocks against VF was 3.3% and the estimated benefit of ICD implantation was of 23% after 2 years 12. Another recent prospective study enrolling a cohort of ARVC patients with an ICD implanted for primary prevention, reported appropriate device interventions in nearly one-half of individuals over a period of 4.7 years 13.
Recommendation for ICD in ARVC
As for IDCM and HCM, ICD implantation is recommended in ARVC for secondary prevention (aborted SCD, VF or hemodynamically unstable sustained VT). ICD is also recommended for primary prevention in patients with LVEF≤35%, severe right ventricular dilation and/or dysfunction, a syncopal episode suggestive for VT or VF or an affected family member with SCD. Other risk factors include: non-sustained VT, early onset of the disease and competitive sport activity.
ICD implantation in ARVC: shadows.
Several studies have proved a relatively high incidence of device-related complications in ARVC patients with an implanted ICD. In a study of 132 patients, five individuals required an additional lead because of pacing failure and one patient died from endocarditis secondary to device infection 14. Progression of fibro-fatty replacement of the myocardial tissue has been associated with high pacing thresholds and impedances and eventual loss of capture. Up to 37% lead-related complication in 7 years have been described in ARVC patients with an implanted ICD 15.
Considerations
The decision regarding ICD implantation is highly significant for any individual at risk of SCD. The clinical decision-making process itself is complex and imply consideration of a number of different aspects. Despite ICD is the only life-prolonging therapy in cardiomyopathies, eventually associated complications should be considered.
Modern technology, such as that utilized by NayaMed, has currently developed sophisticated algorithms allowing an accurate discrimination of supraventricular from ventricular arrhythmic episodes and properly guiding ICD therapies. A growing knowledge of those algorithms by the cardiologists would hopefully reduce the rate of inappropriately delivered shocks by ICDs.
Reliable RV lead alerts and RV lead integrity algorithms, also a constant automatic measurement of leads’ impedances and P, R Waves amplitude have also been implemented in the ICDs of NayaMed, preventing the problems caused by a lead failure. However, despite such algorithms, a complete abolition of ICD-related complications in cardiomyopathies is unreliable. Therefore, an accurate risk stratification appears necessary, as well as a careful weigh of the relative risks and benefits of ICD implantation in each individual.
Dr. Annamaria Martino
Dr. Leonardo Calo
Policlinico Casilino
Roma, Italy
References
1) Gersh J, Maron BJ, Bonow RO et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011; 58:e212-260
2) Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002; 287:1308-1320
3) Maron BJ, Spirito B, Shen WK et al. Implantable cardioverter defibrillators and prevention of sudden cardiac death in hypertrophyc cardiomyopathy. JAMA 2007; 298: 405-412
4) O’Mahony C, Lambiase PD, Quarta G et al. The long-term survival and the risk and benefits of implantable cardioverter defibrillators in patients with hypertrophic cardiomyopathy. Heart 2012;98:116-125
5) Schinkel AF, Vriesendorp PA, Sijbrands EJ et al. Outcome and complications after implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy: systematic review and meta-analysis. Circ Heart Fail 2012;5:552-559
6) Codd MB, Sugrue DD, Gersh BJ et al. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population based study in Olmsted County, Minnesota, 1975–1984. Circulation 1989;80:564–72
7) Bardy GH, Lee KL, Mark DB, Poole JE et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237.
8) Kadish A, Dyer A, Daubert JP et al; Defibrillators in non-ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004;350:2151–2158.
9) Desai AS, Fang JC, Maisel WH, Baughman KL. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trial. JAMA 2004;292: 2874–287
10) Zipes DP; Camm AJ, Borggrefe M et al. ACC/AHA/ESC 2006. Guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2006; 8:746-837
11) Kadish A, Schaechter A, Subacius H et al. Patients with recently diagnosed nonischemic cardiomyopathy benefit from implantable cardioverter defibrillators. J Am Coll Cardiol 2006;47:2477–2482
12) Corrado D et al. Prophylactic Implantable Defibrillator in Patients With Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia and No Prior Ventricular Fibrillation or Sustained Ventricular Tachycardia. Circulation. 2010;122:1144-1152.
13) Bhonsale A, James CA, Tichnell C et al. Incidence and predictors of implantable cardioverter-defibrillator therapy in patients with arrhythmogenic right ventricular dysplasia/ cardiomyopathy undergoing implantable cardioverter-defibrillator implantation for primary prevention. J Am Coll Cardiol, 2011;58:1485-96
14) Corrado D, Leoni L, Link MS et al. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2003;108:3084–91.
15) Wichter T, Paul M,Wollmann C et al. Implantable cardioverter/defibrillator therapy in arrhythmogenic right ventricular cardiomyopathy. Single-center experience of long-term follow-up and complications in 60 patients. Circulation 2004;109:1503–8.
Thank you for visiting us at the ESC Congress 2012 in Munich
Posted: September 10, 2012 Filed under: NayaMed spirit | Tags: bradycardia, cardiac electrophysiology, cardiac techniques, congress, eHealthcare, ESC, Germany, Health 2.0, healthcare organizations, ICD, Medical Devices, Munich, pacemaker, pacemakers, video 4 CommentsNayaMed products in a few words
Posted: August 20, 2012 Filed under: Medical Devices | Tags: bradycardia, cardiac, cardiac electrophysiology, cardiac techniques, cardiology, conventional devices, defibrillator, eHealthcare, Health 2.0, ICD, Medical Devices, pacemaker, pacemakers, St Jude Medical, technical advisor Leave a commentAs a new appearance on the CRDM Market, customers often ask: “Who are you NayaMed?”, “What are your products and why should we chose you for our patients?”
Well, mostly because we offer a simple online platform with a permanent access to your products, giving full transparency and allowing permanent access to ordering, digital inventory, stock management access and technical support.
Also, because as a clinician, you get the product you need when you need. A product that has been designed and engineered to perfectly match the NayaMed philosophy – Smart and Simple.
We wanted the clinicians to be comfortable when using our devices, so we asked our Technical Advisors to present the NayaMed products in just a few minutes.
Mary Soranno and Marina Mancusi, our technical advisors for the UK and the Italian market did a wonderful job and we are inviting you to see a very brief, but complete presentation of our products in English or Italian.
English Version:
Italian Version:
NayaMed devices made simple. Watch it now!
Posted: August 13, 2012 Filed under: Medical Devices, Uncategorized | Tags: bradycardia, cardiac, cardiac electrophysiology, cardiac techniques, cardiology, conventional devices, defibrillator, eHealthcare, Health 2.0, healthcare applications, heart failure, ICD, Medical Devices, pacemaker, pacemakers, video Leave a commentMeet Mary Soranno, our technical advisor for the UK market presenting all the features of the NayaMed pacemakers & defibrillators. (Better than a long blog post or brochure!).
MR conditional devices is progress… and burden
Posted: April 16, 2012 Filed under: Medical Devices | Tags: defibrillator, MR conditional device, MRI, pacemaker, X-ray 5 Comments Last year in November we find out about the launch on the market of the first MR conditional ICD. I must say, I was pleasantly surprised about this and without a doubt the availability of the MR conditional cardiac devices is a step in the right direction.
Last year in November we find out about the launch on the market of the first MR conditional ICD. I must say, I was pleasantly surprised about this and without a doubt the availability of the MR conditional cardiac devices is a step in the right direction.
I knew of course about the availability of MR conditional pacemakers. During a number of years I was even involved in the evaluation and in the launch of MR compatible pacemakers, so, I took a step back and look at the entire picture…
Back in 2005 before the launch of the first MR Conditional pacemaker in the European Market, it was a real believe that this is a breakthrough in the Cardiac Pacing and it will radically change the practice making the MR conditional the new standard for pacemakers.
After 6-7 years we see that although there is progress, a lot of questions and issues are still coming making the entire process sometimes pretty complicate and therefore a limited number of patients really benefit from it, and as Dr. J Rod Gimbel (Cardiology Associates of East Tennessee, Knoxville) mentioned in 2010 in an article published on the “theheart.org” “No one—or company—is suggesting that you replace all of your device patients with an MRI-compatible device” 1
First thing that comes to my mind is the limitation the implanter has in choosing his implantable material. The device and the leads have to be from the same manufacturer and both labelled MR conditional. Only a very limited number of lead models are available, some with an MR Conditional X-Ray ID, some not. Also, in one manufacturer, the combination of two lead models, both MR conditional, in a single device system is not tested MR conditional2.
Another aspect is the replacement (box change) process, which can be difficult; as an MR conditional device implanted over a non-MR conditional lead, makes the entire system NON MR conditional. The solution would be the removal of any implanted lead and its replacement with a new MR conditional one, but this will bring additional risks.
From the radiologist point of view it doesn’t became simpler as some devices have an exclusion zone and also a limitation in the absorption rate which is different from a device to another.
Concerning the exclusion zone, having a photo of the body with the marked zones it is really useful for the practitioner. Looking a little closer to the exclusion zones and the permissible zones of one manufacturer, I realised a significant part of the body can’t be scanned, practically the entire spine, the entire chest, elbows and a good part of the arms3.
By doing some research over the Internet I found an article about the growth rate of the MRI procedure and more interesting, about the MRI procedure Mix in 2007 by anatomy. According to this table, 26% of the MRI procedures are for Spine, for the Chest 2%, Breast 2%, Cardiac 1%, Upper extremities 9%, pelvic and abdominal 8%4. If I do the math and I look again at the exclusion zone picture, I almost could roughly say, 40% of the body can’t be scanned.
From my knowledge there is not yet a standard procedure among radiologists for the scan of an MR Conditional device, taking in consideration all the differences between different manufacturers.
Well, it’s true that 2 out of the 3 manufacturers do not have exclusion zones and this is a clear advantage but they are all limited to 1.5 Tesla machines only, not more and not less. The MRI technology is advancing to 3 Tesla machines and today, in 3 Tesla none of the devices is MR conditional.
We are part of the medical device industry and as I pointed in the beginning of my article, advancing towards MR Safe devices is the good direction. For the moment, considering the aspects I mentioned, I think the MR conditional devices are not yet simply and straight forward to implant and specially to scan safely.
Those reasons and probably the price uplift the manufacturers are setting for this new technology are the reasons why, after a number of years since those devices are on the market only 10 to 25 % of the implanted devices are MR conditional.
Having mentioned that, in NayaMed we choose to offer devices the doctor will choose to implant in the big majority of all the device patients and not only in 10 to 25 % of them. Simple and fit for purpose devices, is what we believe in, and for the moment, in our opinion, MR conditional technology doesn’t fit in this category.
Two weeks ago I did a knee MRI, because the X-Ray didn’t show anything and I was still having knee pain. I couldn’t help myself thinking “If I had a pacemaker or an ICD implanted I would like-it to be MR conditional… the knee is outside any exclusion zone”
One week later I read the procedure report. The doctor found 2 tears in my meniscus, thanks to this MRI procedure… performed on a 3 Tesla Machine. Actually… 3 out of 4 machines in the biggest hospital of my region are 3 Tesla5.
Alexandru Trif
Product Manager @NayaMed
- http://www.theheart.org/article/1059397.do (accessed on the 30th of March 2012)
- http://manuals.biotronik.com/sixcms/media.php/174/iHB_ProMRI_en_371712-J_2011-11-22.pdf (accessed on the 30th of March 2012)
- http://manuals.biotronik.com/sixcms/media.php/174/iHB_ProMRI_en_371712-J_2011-11-22.pdf (accessed on the 30th of March 2012)
- http://www.auntminnie.com/index.aspx?sec=sup&sub=bai&pag=dis&ItemId=81355 (accessed on the 30th of March 2012)
- http://www.chuv.ch/rad/rad_home/rad-organisation/rad-organisation-radiologie/rad-organisation-radiologie-equipement/rad-organisation-radiologie-equipement-irm.htm (accessed on the 30th of March 2012)