Cardiac conduction disturbances : the other side of sudden cardiac death in hypertrophic cardiomyopathy.
Posted: October 21, 2013 Filed under: Medical Devices, Professionnal guest | Tags: arrhythmias, atrio-ventricular block, AVB, cardiac, cardiac conduction disturbances, cardiac death, cardio, cardiomyopathy, CCD, HCM, hypertrophic, Hypertrophic cardiomyopathy, myocardial, sudden, sudden cardiac death, tachy-arrhythmias, ventricular Leave a commentCardiac conduction disturbances: the other side of sudden cardiac death in hypertrophic cardiomyopathy.
Hypertrophic cardiomyopathy (HCM) is a myocardial disease characterized by asymmetrical hypertrophy of the heart and disarray of myocardial cells, with an unstable electrical substrate potentially causing lethal ventricular tachy-arrhythmias 1.
Sudden cardiac death is the worst feared complication of hypertrophic cardiomyopathy (HCM), and is known to be mostly related to ventricular arrhythmias such as ventricular fibrillation and ventricular tachycardia 1. However, arrhythmic sudden cardiac death can also be related to cardiac conduction disturbances (CCD), including complete atrio-ventricular block (AVB), possibly leading to prolonged asystolia. The only available treatment for such CCD, is the pacemaker (PM).
Third degree atrio-ventricular block.
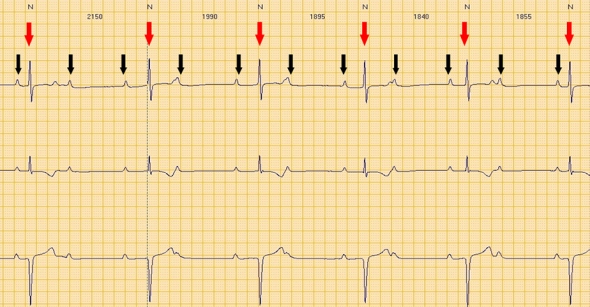
Red arrows: QRS (ventricular electrical activity). Black arrows: P waves (atrial electrical activity).
Atrial and ventricular activity is completely dissociated.
Second degree advanced atrio-ventricular block.
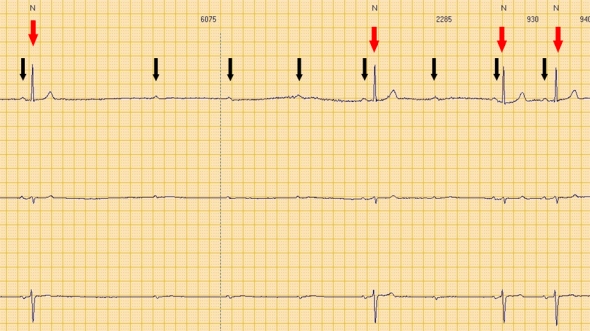
Red arrows: QRS (ventricular electrical activity). Black arrows: P waves (atrial electrical activity).
Consecutive P waves care not followed by QRS, leading to a transient asystolic pause.
Although CCD are commonly seen in association with amyloidosis and glycogen storage disorders, which may themselves be associated with left ventricular hypertrophy 1, little is known about the prevalence of primitive CCD in HCM.
Primitive CCD
Case reports.
A few case reports of HCM patients with complete primitive (i.e. not secondary to surgicalor interventional procedures) AVB have been reported.
Paroxysmal complete AVB has been described in three HCM patients, with prolonged QRS duration at ECG, suffering from recurrent attacks of syncope and cardiopulmonary arrest 2. Symptoms were likely related to the AVB itself.
A case of a north Indian family including nine HCM individuals, among whom 5 underwent PM implantation due to advanced AVB at an age of 39-55 years, has also been described 3. Two sudden cardiac deaths (25 and 51 years of age respectively) were also reported in the same family. The absence of CCD in all the HCM-affected members of the family (therefore sharing the same genetic mutation, likely associated with CCD), may be explained by the phenomenon of incomplete “penetrance” (i.e. the proportion of individuals with the mutation who exhibit clinical symptoms), which in turn is influenced by several factors, including age and gender.
Clinical studies
Prospective studies investigating the prevalence of CCD in HCM requiring permanent PM implantation are lacking. In a retrospective analysis of a small cohort of HCM patients (27 patients; 12 non obstructive) with an implanted PM, a high rate of AVB was observed4. Indications for PM included: spontaneous or induced AVB in 54% of cases (with spontaneous total AVB in over 15% of cases) and support for drug-induced bradycardia in 8% of cases 4.
A larger retrospective study of 451 HCM-patients (44% with previous syncope), also reported a high prevalence of CCD. Overall, PM was implanted in 11% of patients, either due to sinus node dysfunction or to AVB. Interestingly, in 18% of cases, at least one other family member was a PM recipient. One single mutation (E101K in the cardiac actin gene) was identified in 3 index cases (PM recipients). The authors concluded that CCD may form part of the phenotype expression of HCM and may have a familial component 5.
Iatrogenic CCD
Obstruction of the LV outflow tract in HCM, due to septal hypertrophy, is dynamic and varies with loading conditions and contractility of the left ventricle. Interventional procedures for reduction of the left ventricular outflow pressure gradient, such as septal alcohol ablation (SA) or surgical septal myectomy (SM) can be complicated by a not negligible rate of CCDs.
Septal ablation
Trans-catheter alcohol embolization of selected branches of the coronary arteries, aims at generating a circumscribed septal infarction of the left ventricle, in an attempt to reduce the left ventricular outflow tract pressure gradient due to loss of myocardial contraction. The iatrogenic septal infarction usually extends between the anterior and inferior free walls of the left ventricle, in an area commonly containing the right bundle branch (RBB) of the conduction system. Septal Ablation is therefore often complicated by RBB block occurrence 6. AVB has also been described in up to 62% of cases of SA, but is usually transient and characterized by a spontaneous regression within 24 hours. The frequency of CCDs following SA which require permanent PM implantation has ranged between 10% and 33% across studies 6.
Subacute AVB have been also been described, occurring up to 8 days after SA 7. Predictors of subacute AVB post-ASA include: advanced age, prolonged QRS duration before or after SA, new intra-ventricular conduction disturbances or first-degree AV block after SA 7.
Septal myectomy
Septal myectomy entails surgical removal of sub-endocardial tissue in the anterior inter-ventricular septum in an attempt to reduce ventricular contractility and left ventricular outflow tract gradient. Since the left bundle branch fibers of the cardiac conduction system are adjacent to the anterior inter-ventricular septum, patients undergoing SM are at increased risk of developing left bundle branch block. Interestingly, CCDs have been shown to be significantly lower following SM as compared with SA 6,8. The risk of complete AVB is approximately 2% with SM (higher in patients with preexisting RBB block 1 .
Considerations
Current guidelines underline the importance of PM implantation when an advanced CCD occurs after SA or SM 1. However, in planning a PM implantation due to AVB in a HCM-patient, a few considerations must be taken into account, including:
- eventual benefits expected from pacing of the right ventricular apex, in terms of reduction of the left ventricular outflow tract gradient and consequent symptoms reduction1;
- the individual risk of tachy-arrhythmias eventually precipitating sudden cardiac death, with possible indication for cardioverter defibrillator implant.
Nowadays, the availability of sophisticated high-quality PM, such as those provided by NayaMed, allows safe and efficient protection from CCD and associated risk of sudden cardiac death in HCM individuals.
Dr. Annamaria Martino, Dr. Lucia De Luca and Prof. Leonardo Calò
Cardiology Department, Policlinic Casilino, ASL RMB, Rome, Italy.
References
- Gersh J. et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011; 58:e212-260
- Doven O et al. Abnormal His- Purkinje system conduction leading to complete atrioventricular block in patients with hypertrophic cardiomyopathy: a report of 3 cases. Jpn Heart J. 2004;45:347-52.
- Bahl A, Nahar Saikia U, Talwar KK. Familial conduction system disease associated with hypertrophic cardiomyopathy. International Journal of Cardiology 2008; 125: e44–e47
- Alves Silva LA et al. Cardiac Pacing in Hypertrophic Cardiomyopathy. A Cohort with 24 Years of Follow-Up. Arq Bras Cardiol 2008;91:250-256
- Barriales-Villa R et al. Severe cardiac conduction disturbances and Pacemaker implantation in patients with hypertrophic cardiomyopathy. Rev Esp Cardiol. 2010;63:985-8
- Agarwal S et al. Updated meta-analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J Am Coll Cardiol 2010;55:823–34
- Lawrenz T et al. Predictors of complete heart block after transcoronary ablation of septal hypertrophy: results of a prospective electrophysiological investigation in 172 patients with hypertrophic obstructive cardiomyopathy. Am Coll Cardiol. 2007;49:2356-63.
- Talreja DR et al. Alcohol septal ablation versus surgical septal myectomy: comparison of effects on atrioventricular conduction tissue. J Am Coll Cardiol.2004;44:2329-32.
Lights and shadows of implantable cardioverter defibrillators implantations in hypertrophic, dilated and arrhythmogenic right ventricular cardiomyopathies.
Posted: April 29, 2013 Filed under: Medical Devices, Opinions | Tags: bradycardia, defibrillator, eHealthcare, Health 2.0, ICD, implantable cardioverter defibrillators, lights, Medical Devices, pacemaker, SCD, sudden cardiac death Leave a commentIn the past decades, implantable cardioverter defibrillators (ICDs) have definitively proved to be superior to antiarrhythmic drugs for the prevention of arrhythmic sudden cardiac death (SCD). Several studies have proved this hypothesis both for primary or secondary (i.e. after a sustained ventricular tachycardia with hemodynamic compromise or ventricular fibrillation) prevention of SCD. However, some complications often occur with ICD implantation, which may be particularly serious for young individuals.
Indications for ICD implantation in young people are mostly ion channel diseases and cardiomyopathies. The latter are structural abnormalities of the myocardium, related to genetic abnormalities, predisposing to malignant ventricular arrhythmias and requiring ICD for the prevention of SCD in some cases.
Hypertrophic cardiomyopathy
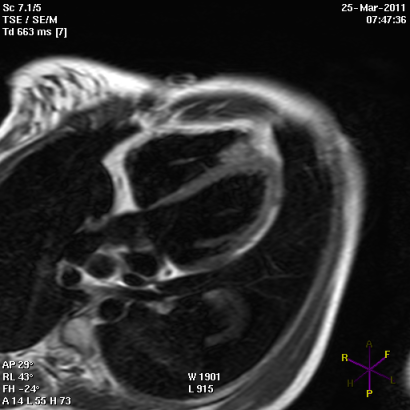
Hypertrophic cardiomyopathy (HCM) is one of the most common cardiomyopathies and occurs in the 0.2% of the general population1. It is characterized by localized asymmetrical hypertrophy of the heart and disarray of myocardial cells and filaments. This leads to impaired transmission of electrophysiologic impulses, with an unstable electrical substrate, potentially causing lethal ventricular tachyarrhythmias and SCD.
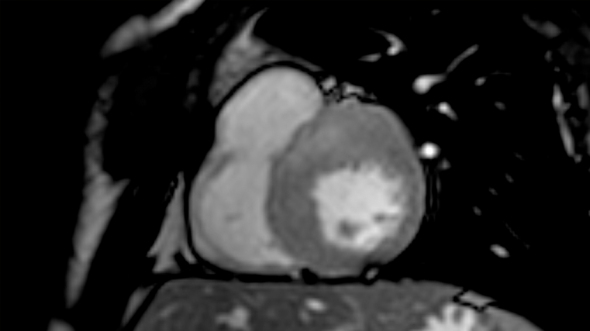
Short axis view of a cardiac magnetic resonance imaging of a patient affected by hypertrophic cardiomyopathy involving the interventricular septum and the anterior wall of the left ventricle.
ICD implantation in HCM: lights
A minority of patients with HCM are judged to be at increased risk for SCD, whose rate is estimated to be about 1% per year 2. However, malignant ventricular arrhythmias remains the most frequent cause of death in this population.
Beta-blockers have failed to demonstrate significant protection from SCD in HCM patients. Type I and III anti-arrhythmic drugs, including amiodarone, have been abandoned because of inefficacy and pro-arrhythmic side effects. Experiences with endocardial and epicardial mapping and ablation in HCM are poor and limited to highly selected patients.
ICDs, conversely, have been proved effective in terminating life-threatening ventricular tachyarrhythmias in HCM and are currently considered the only available tools altering the natural course of the disease and prolonging life.
Appropriate intervention rates of ICD in HCM have been esteemed to be about 11% and 4% for secondary and primary prevention respectively3.
Current recommendation for ICD in HCM
HCM has heterogeneous clinical manifestations in different individuals and a not well predictable clinical course. Targeting HCM-patients for prophylactic ICD implantation can therefore be challenging. However, some “risk factors” have been individuated, that commonly guide the decision for ICD implantation. Current international guidelines recommend ICD in all cases of secondary prevention or when familiar history of SCD, marked left ventricular (LV) hypertrophy or recent unexplained syncopal episodes are present. The role of ICD is uncertain for non-sustained VT or an abnormal blood pressure response with exercise.
ICD implantation in HCM: shadows
Previous studies have reported up to 5.1%/year rate of ICD-related complications in HCM4.
The most frequently reported is inappropriate shock delivery, mostly due to erroneous detection and treatment of atrial fibrillation by the ICD. A retrospective study on 334 consecutive HCM patients with an ICD, showed a rate of appropriate vs. inappropriate shock delivery of 2.3 and 4.6%/year5. Moreover, a recent meta-analysis involving 2190 ICD-recipients affected by HCM, showed a rate of appropriate vs. inappropriate ICD interventions of 3.3 vs. 4.8%/year respectively4.
Another extremely rare complication with ICDs in HCM, is represented by loss of capture due to high pacing thresholds (i.e. energy required for effective right ventricular pacing). This is related to ventricular hypertrophy and may be prevented by accurate individuation of the optimal pacing threshold during ICD programming.
Lengthened mitral valve leaflets, and left ventricular outflow tract obstruction due to LV hypertrophy, also confers a relatively higher risk of infection and endocarditis to HCM patients, which must be considered when an ICD implantation is programmed. Moreover, since ICD implantation in HCM is commonly performed at a relatively young age, patients are supposed to necessitate of a number of interventions for pulse generator substitution over their entire life. For this reason, risk of infection and endocarditis (related to ICD substitutions) is amplified. Eventual needing of ICD and intra-cardiac leads extraction over time must be considered. This is a high-risk procedure, possibly complicated by cardiac tamponade, shock, anemia, arrhythmias or even death.
Finally, ICD implantation can be accompanied by depression, anxiety, reduced quality of life, particularly in young people.
Idiopathic dilated cardiomyopathy
Idiopathic dilated cardiomyopathy (IDCM) is a myocardial disease characterized by LV dilation and systolic dysfunction, commonly resulting in heart failure (HF) and for whom an etiological basis cannot be identified. IDCM is relatively rare (36.5% new cases/year/100.000 persons) but accounts for nearly 10.000 deaths/year in the United States, both due to HF and arrhythmic SCD6.
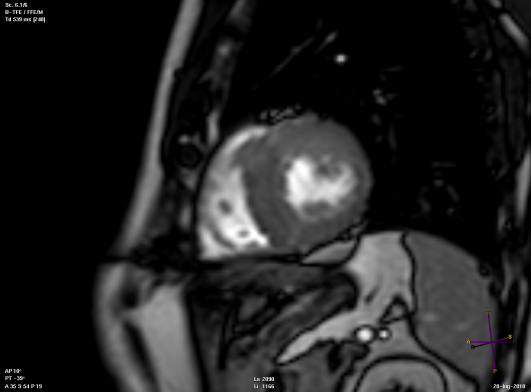
Long axis view of a cardiac magnetic resonance imaging of a patient affected by dilated cardiomyopathy
ICD implantation in IDCM: lights
ICD are effective for the prevention of SCD in IDCM and can favorably alter the natural course of the disease. Previous studies have showed a rate of appropriate interventions of 5 to 7.5%/year in IDCM-patients with an ICD implanted for primary prevention 7-8. A large meta-analysis has showed a reduction of mortality with ICD of about 3.5%/year compared to the best medical therapy in IDCM 9.
Recommendation for ICD in IDCM
The most important risk factor for ventricular arrhythmias in primary prevention of SCD in IDCM, is represented by severe contractile dysfunction, quantified by the measurement of the ejection fraction (EF) at echocardiography. Functional status, assessed by the New York Heart Association (NYHA) class, also plays a role.
Current international guidelines recommend ICD always for secondary prevention. In case of primary prevention, ICD must be implanted in patients with LVEF ≤35% and NYHA class II and III10. However, in a real-life setting, the decision making for patients with IDCM is more complex, because issues such as additional cardiac resynchronization therapy (CRT), co-morbidities, the potential to improve LVEF over time, and eventual genetic etiology also should be considered. With regard to the last issue, carriers of mutations in gene encoding for laminin, are tough to be at increased risk of complete atrio-ventricular block, malignant ventricular arrhythmias and SCD, and may therefore require ICD implantation independently from the EF%.
ICD implantation in IDCM: shadows and warnings
Some shadows obscure the scenario of SCD prevention with ICDs in IDCM.
An important issue regards the high number of patients to be treated in order to save one life because of a currently adopted risk stratification process that appears to lack specificity.
Another topic is the correct timing for ICD implantation for primary prevention. A post hoc analysis of the DEFINITE trials 11 showed that only patients who had received their ICD not later than 3 months after the diagnosis of IDCM would have benefit from implant. However, a significant number of IDCM-individuals show marked improvement of the EF over time, up to values higher than those for which ICD implantation is currently recommended. The goal therefore, appears to be an early and correct individuation of those subjects who will have a negative clinical course, and will therefore require an early ICD implantation for primary prevention.
Similarly to HCM, ICD-related complications have been also reported by various studies, among whom:
- inappropriate shocks, with consequent reduction of quality of life;
- infections;
- risks related to eventual lead extractions;
- depression and anxiety.
Arrhythmogenic right ventricular cardiomyopathy
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a genetic cardiomyopathy characterized by progressive fibro-fatty replacement of the right ventricular myocardium, sometimes extending also to the left ventricle. The clinical presentation is usually related to ventricular tachycardia (VT) with a left bundle branch block pattern or ventricular fibrillation (VF) leading to SCD. ARVC is a progressive disease ultimately leading to HF.
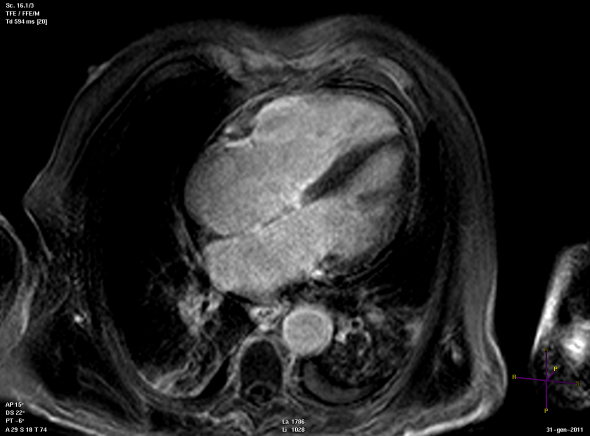
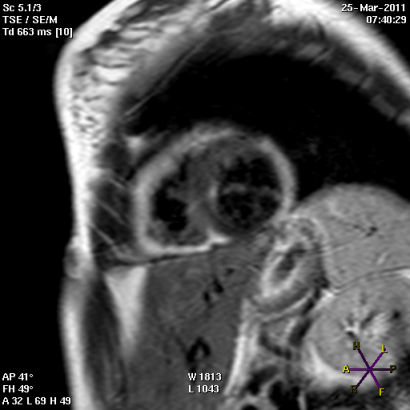
Panel A
Panel B
Short (Panel A) and long axis views (panel B) of a cardiac magnetic resonance imaging of a patient affected by arrhythmogenic right ventricular cardiomyopathy, with evident fibro-fatty replacement of the right ventricle wall, the apex and partial involvement of the left ventricle wall.
ICD implantation in ARVC: lights
The therapeutic options for ventricular arrhythmias in ARVC include catheter ablation and antiarrhythmic drugs such as beta blockers, sotalol and amiodarone. However, these strategies have proved to improve symptoms but not to increase survival. Nowadays, ICD remains the most effective safe-guard against SCD in ARVC.
In a large multicenter international study enrolling ARVC patients with an ICD implanted for primary prevention, appropriate device interventions were observed in one fourth of patients after 5 years. The annual rate of potentially “life-saving” shocks against VF was 3.3% and the estimated benefit of ICD implantation was of 23% after 2 years 12. Another recent prospective study enrolling a cohort of ARVC patients with an ICD implanted for primary prevention, reported appropriate device interventions in nearly one-half of individuals over a period of 4.7 years 13.
Recommendation for ICD in ARVC
As for IDCM and HCM, ICD implantation is recommended in ARVC for secondary prevention (aborted SCD, VF or hemodynamically unstable sustained VT). ICD is also recommended for primary prevention in patients with LVEF≤35%, severe right ventricular dilation and/or dysfunction, a syncopal episode suggestive for VT or VF or an affected family member with SCD. Other risk factors include: non-sustained VT, early onset of the disease and competitive sport activity.
ICD implantation in ARVC: shadows.
Several studies have proved a relatively high incidence of device-related complications in ARVC patients with an implanted ICD. In a study of 132 patients, five individuals required an additional lead because of pacing failure and one patient died from endocarditis secondary to device infection 14. Progression of fibro-fatty replacement of the myocardial tissue has been associated with high pacing thresholds and impedances and eventual loss of capture. Up to 37% lead-related complication in 7 years have been described in ARVC patients with an implanted ICD 15.
Considerations
The decision regarding ICD implantation is highly significant for any individual at risk of SCD. The clinical decision-making process itself is complex and imply consideration of a number of different aspects. Despite ICD is the only life-prolonging therapy in cardiomyopathies, eventually associated complications should be considered.
Modern technology, such as that utilized by NayaMed, has currently developed sophisticated algorithms allowing an accurate discrimination of supraventricular from ventricular arrhythmic episodes and properly guiding ICD therapies. A growing knowledge of those algorithms by the cardiologists would hopefully reduce the rate of inappropriately delivered shocks by ICDs.
Reliable RV lead alerts and RV lead integrity algorithms, also a constant automatic measurement of leads’ impedances and P, R Waves amplitude have also been implemented in the ICDs of NayaMed, preventing the problems caused by a lead failure. However, despite such algorithms, a complete abolition of ICD-related complications in cardiomyopathies is unreliable. Therefore, an accurate risk stratification appears necessary, as well as a careful weigh of the relative risks and benefits of ICD implantation in each individual.
Dr. Annamaria Martino
Dr. Leonardo Calo
Policlinico Casilino
Roma, Italy
References
1) Gersh J, Maron BJ, Bonow RO et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011; 58:e212-260
2) Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002; 287:1308-1320
3) Maron BJ, Spirito B, Shen WK et al. Implantable cardioverter defibrillators and prevention of sudden cardiac death in hypertrophyc cardiomyopathy. JAMA 2007; 298: 405-412
4) O’Mahony C, Lambiase PD, Quarta G et al. The long-term survival and the risk and benefits of implantable cardioverter defibrillators in patients with hypertrophic cardiomyopathy. Heart 2012;98:116-125
5) Schinkel AF, Vriesendorp PA, Sijbrands EJ et al. Outcome and complications after implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy: systematic review and meta-analysis. Circ Heart Fail 2012;5:552-559
6) Codd MB, Sugrue DD, Gersh BJ et al. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population based study in Olmsted County, Minnesota, 1975–1984. Circulation 1989;80:564–72
7) Bardy GH, Lee KL, Mark DB, Poole JE et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237.
8) Kadish A, Dyer A, Daubert JP et al; Defibrillators in non-ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004;350:2151–2158.
9) Desai AS, Fang JC, Maisel WH, Baughman KL. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trial. JAMA 2004;292: 2874–287
10) Zipes DP; Camm AJ, Borggrefe M et al. ACC/AHA/ESC 2006. Guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2006; 8:746-837
11) Kadish A, Schaechter A, Subacius H et al. Patients with recently diagnosed nonischemic cardiomyopathy benefit from implantable cardioverter defibrillators. J Am Coll Cardiol 2006;47:2477–2482
12) Corrado D et al. Prophylactic Implantable Defibrillator in Patients With Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia and No Prior Ventricular Fibrillation or Sustained Ventricular Tachycardia. Circulation. 2010;122:1144-1152.
13) Bhonsale A, James CA, Tichnell C et al. Incidence and predictors of implantable cardioverter-defibrillator therapy in patients with arrhythmogenic right ventricular dysplasia/ cardiomyopathy undergoing implantable cardioverter-defibrillator implantation for primary prevention. J Am Coll Cardiol, 2011;58:1485-96
14) Corrado D, Leoni L, Link MS et al. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2003;108:3084–91.
15) Wichter T, Paul M,Wollmann C et al. Implantable cardioverter/defibrillator therapy in arrhythmogenic right ventricular cardiomyopathy. Single-center experience of long-term follow-up and complications in 60 patients. Circulation 2004;109:1503–8.