Shock reduction using ATP as 1st line therapy for spontaneous sustained ventricular tachycardia in patients with ischemic dilated cardiomyopathy and severly depressed ventricular function
Posted: September 26, 2014 Filed under: eHealthCare, Medical Devices, Professionnal guest Leave a commentAuthors: Stefano Nardi MD, PhD (*), Luigi Argenziano MD, PhD (*)
(*) Arrhythmia, Electrophysiologic Center and Cardiac Pacing Unit, Thoracic Surgery and Cardiovascular Department, Presidio Ospedaliero Pineta Grande, Castel Volturno (CE), Italy (I)
Introduction
Implantable cardioverter-defibrillators (ICDs) have clearly demonstrated to terminate an elevated percentage of sustained ventricular tachycardia (SVTs), both with shock therapy and, painlessly, with antitachycardia pacing (ATP). The purpose of this study was to investigate the efficacy and safety of ATP as 1st line therapy for terminating SVTs, occurring in patients (pts) with coronary artery disease (CAD) and severely depressed left ventricular ejection fraction (LVEF) due to not reversible causes, comparing this approach with shock.
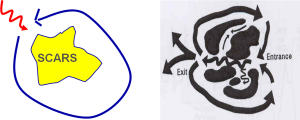
Figure 1: Rationale for ATP Rx is that a “WAVE-FRONT” critically temporized with VTCL and applied proximal to the “Slow Conduction Zone” is able to capture the CIRCUIT, then interrupt the reentry
Methods
From March 2004 through June 2013, 408 consecutive patients (pts) were considered eligible for this study, according with MADIT and/or SCD-HeFT criteria, and 387 of them (94,8%) accepted and underwent ICD implant. Pts were randomly assigned to ATP group (190/387) or shock group (197/387) and three zone of detection were assigned in all pts: a slow VTs window (320-400 msec), a fast VTs window (240-320 msec), and a ventricular fibrillation (VF) window (<320 msec). In ATP group, 3 burst sequence were programmed in slow VT zone and 1 burst sequence was programmed in fast VTs zone (burst sequences of 8-10 pulses at 88% CL) before shock, respectively, whereas in shock group (87/169) shock was adopted as 1st line therapy for all SVTs. No statistically differences, in term of age (72,2 vs 73,4, p=NS), sex (man 73,1% vs 69,3%, p=NS), NYHA functional class (2,8 vs 2,6, p=NS), LVEF (24,5% vs 26,9%) and drugs therapy were observed between two groups. Based on the evaluation of stored electrograms (EGMs), we aimed to prospectively follow pts and to analyze these two different therapeutic approach.
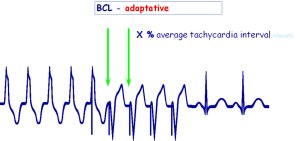
Figure 2: ATP programmability with adaptative cycle length
Results
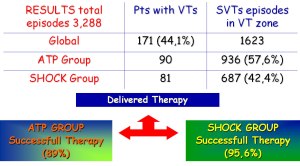
A total of 3,288 spontaneous VTs episodes occurred in 171 pts (41,9%) during a mean follow-up of 54,4±23,1 months (90 pts in ATP group and 81 pts in shock group). Among these, 2,235/3,288 (67,9%) were SVTs and 612 of them (27,4%) were detected in VF zone, and then treated with primary shock delivery, whereas in the remaining 1,623 of them (72,6%), 936 episodes occurred in ATP group (57,6%) and 687 episodes occurred in shock group (42,4%). In ATP group, ATP was able to terminate successfully 80,7% of SVTs, failed in 9,3% of SVTs, finally reverted by shocks, and in 10% of SVTs ATP was able to converted arrhythmias in a slower VT, outside the VT zone. 61% of these slower SVTs were self-terminating whereas 39% were redetected and treated. Finally, in primary intention-to-treat basis, ATP was successful in 834/936 SVTs (89%) and unsuccessful in 102/936 SVTs (11%). In contrast, 657/687 (95,6%) of all SVTs episode detected in VT zone and that occur in shock group were shock terminated, whereas 30/657 (4,4%) were accelerated into VF zone. The individual termination rate and acceleration rate per pt were comparable in both groups, as well as mean time of SVT termination (ATP=12.6 sec vs Shock=10.9 sec, p=NS).
Figure 4: Results of a registry comparing ATP vs shock therapy as primary choice of VT treatment for a total of 387 pts enrolled
Conclusions
Our data suggest that ATP is a safe and effective therapeutic approach for SVTs termination, also in pts with DCM and very depressed LVEF; then, this therapeutic approach should be programmed “on” in all patients regardless LVEF.
Authors: Stefano Nardi MD, PhD (*), Luigi Argenziano MD, PhD (*)
(*) Arrhythmia, Electrophysiologic Center and Cardiac Pacing Unit, Thoracic Surgery and Cardiovascular Department, Presidio Ospedaliero Pineta Grande, Castel Volturno (CE), Italy (I)
Cardiac conduction disturbances : the other side of sudden cardiac death in hypertrophic cardiomyopathy.
Posted: October 21, 2013 Filed under: Medical Devices, Professionnal guest | Tags: arrhythmias, atrio-ventricular block, AVB, cardiac, cardiac conduction disturbances, cardiac death, cardio, cardiomyopathy, CCD, HCM, hypertrophic, Hypertrophic cardiomyopathy, myocardial, sudden, sudden cardiac death, tachy-arrhythmias, ventricular Leave a commentCardiac conduction disturbances: the other side of sudden cardiac death in hypertrophic cardiomyopathy.
Hypertrophic cardiomyopathy (HCM) is a myocardial disease characterized by asymmetrical hypertrophy of the heart and disarray of myocardial cells, with an unstable electrical substrate potentially causing lethal ventricular tachy-arrhythmias 1.
Sudden cardiac death is the worst feared complication of hypertrophic cardiomyopathy (HCM), and is known to be mostly related to ventricular arrhythmias such as ventricular fibrillation and ventricular tachycardia 1. However, arrhythmic sudden cardiac death can also be related to cardiac conduction disturbances (CCD), including complete atrio-ventricular block (AVB), possibly leading to prolonged asystolia. The only available treatment for such CCD, is the pacemaker (PM).
Third degree atrio-ventricular block.
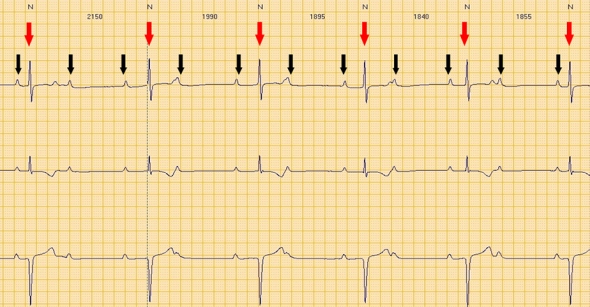
Red arrows: QRS (ventricular electrical activity). Black arrows: P waves (atrial electrical activity).
Atrial and ventricular activity is completely dissociated.
Second degree advanced atrio-ventricular block.
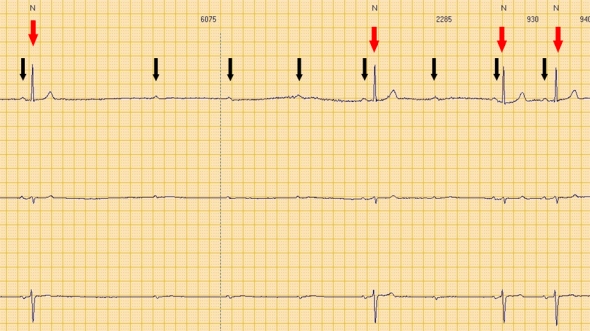
Red arrows: QRS (ventricular electrical activity). Black arrows: P waves (atrial electrical activity).
Consecutive P waves care not followed by QRS, leading to a transient asystolic pause.
Although CCD are commonly seen in association with amyloidosis and glycogen storage disorders, which may themselves be associated with left ventricular hypertrophy 1, little is known about the prevalence of primitive CCD in HCM.
Primitive CCD
Case reports.
A few case reports of HCM patients with complete primitive (i.e. not secondary to surgicalor interventional procedures) AVB have been reported.
Paroxysmal complete AVB has been described in three HCM patients, with prolonged QRS duration at ECG, suffering from recurrent attacks of syncope and cardiopulmonary arrest 2. Symptoms were likely related to the AVB itself.
A case of a north Indian family including nine HCM individuals, among whom 5 underwent PM implantation due to advanced AVB at an age of 39-55 years, has also been described 3. Two sudden cardiac deaths (25 and 51 years of age respectively) were also reported in the same family. The absence of CCD in all the HCM-affected members of the family (therefore sharing the same genetic mutation, likely associated with CCD), may be explained by the phenomenon of incomplete “penetrance” (i.e. the proportion of individuals with the mutation who exhibit clinical symptoms), which in turn is influenced by several factors, including age and gender.
Clinical studies
Prospective studies investigating the prevalence of CCD in HCM requiring permanent PM implantation are lacking. In a retrospective analysis of a small cohort of HCM patients (27 patients; 12 non obstructive) with an implanted PM, a high rate of AVB was observed4. Indications for PM included: spontaneous or induced AVB in 54% of cases (with spontaneous total AVB in over 15% of cases) and support for drug-induced bradycardia in 8% of cases 4.
A larger retrospective study of 451 HCM-patients (44% with previous syncope), also reported a high prevalence of CCD. Overall, PM was implanted in 11% of patients, either due to sinus node dysfunction or to AVB. Interestingly, in 18% of cases, at least one other family member was a PM recipient. One single mutation (E101K in the cardiac actin gene) was identified in 3 index cases (PM recipients). The authors concluded that CCD may form part of the phenotype expression of HCM and may have a familial component 5.
Iatrogenic CCD
Obstruction of the LV outflow tract in HCM, due to septal hypertrophy, is dynamic and varies with loading conditions and contractility of the left ventricle. Interventional procedures for reduction of the left ventricular outflow pressure gradient, such as septal alcohol ablation (SA) or surgical septal myectomy (SM) can be complicated by a not negligible rate of CCDs.
Septal ablation
Trans-catheter alcohol embolization of selected branches of the coronary arteries, aims at generating a circumscribed septal infarction of the left ventricle, in an attempt to reduce the left ventricular outflow tract pressure gradient due to loss of myocardial contraction. The iatrogenic septal infarction usually extends between the anterior and inferior free walls of the left ventricle, in an area commonly containing the right bundle branch (RBB) of the conduction system. Septal Ablation is therefore often complicated by RBB block occurrence 6. AVB has also been described in up to 62% of cases of SA, but is usually transient and characterized by a spontaneous regression within 24 hours. The frequency of CCDs following SA which require permanent PM implantation has ranged between 10% and 33% across studies 6.
Subacute AVB have been also been described, occurring up to 8 days after SA 7. Predictors of subacute AVB post-ASA include: advanced age, prolonged QRS duration before or after SA, new intra-ventricular conduction disturbances or first-degree AV block after SA 7.
Septal myectomy
Septal myectomy entails surgical removal of sub-endocardial tissue in the anterior inter-ventricular septum in an attempt to reduce ventricular contractility and left ventricular outflow tract gradient. Since the left bundle branch fibers of the cardiac conduction system are adjacent to the anterior inter-ventricular septum, patients undergoing SM are at increased risk of developing left bundle branch block. Interestingly, CCDs have been shown to be significantly lower following SM as compared with SA 6,8. The risk of complete AVB is approximately 2% with SM (higher in patients with preexisting RBB block 1 .
Considerations
Current guidelines underline the importance of PM implantation when an advanced CCD occurs after SA or SM 1. However, in planning a PM implantation due to AVB in a HCM-patient, a few considerations must be taken into account, including:
- eventual benefits expected from pacing of the right ventricular apex, in terms of reduction of the left ventricular outflow tract gradient and consequent symptoms reduction1;
- the individual risk of tachy-arrhythmias eventually precipitating sudden cardiac death, with possible indication for cardioverter defibrillator implant.
Nowadays, the availability of sophisticated high-quality PM, such as those provided by NayaMed, allows safe and efficient protection from CCD and associated risk of sudden cardiac death in HCM individuals.
Dr. Annamaria Martino, Dr. Lucia De Luca and Prof. Leonardo Calò
Cardiology Department, Policlinic Casilino, ASL RMB, Rome, Italy.
References
- Gersh J. et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011; 58:e212-260
- Doven O et al. Abnormal His- Purkinje system conduction leading to complete atrioventricular block in patients with hypertrophic cardiomyopathy: a report of 3 cases. Jpn Heart J. 2004;45:347-52.
- Bahl A, Nahar Saikia U, Talwar KK. Familial conduction system disease associated with hypertrophic cardiomyopathy. International Journal of Cardiology 2008; 125: e44–e47
- Alves Silva LA et al. Cardiac Pacing in Hypertrophic Cardiomyopathy. A Cohort with 24 Years of Follow-Up. Arq Bras Cardiol 2008;91:250-256
- Barriales-Villa R et al. Severe cardiac conduction disturbances and Pacemaker implantation in patients with hypertrophic cardiomyopathy. Rev Esp Cardiol. 2010;63:985-8
- Agarwal S et al. Updated meta-analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J Am Coll Cardiol 2010;55:823–34
- Lawrenz T et al. Predictors of complete heart block after transcoronary ablation of septal hypertrophy: results of a prospective electrophysiological investigation in 172 patients with hypertrophic obstructive cardiomyopathy. Am Coll Cardiol. 2007;49:2356-63.
- Talreja DR et al. Alcohol septal ablation versus surgical septal myectomy: comparison of effects on atrioventricular conduction tissue. J Am Coll Cardiol.2004;44:2329-32.