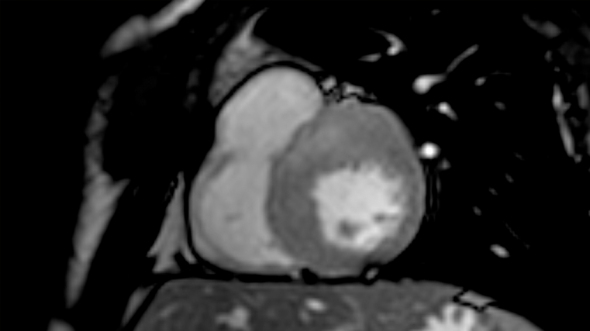
Cardiac conduction disturbances : the other side of sudden cardiac death in hypertrophic cardiomyopathy.
Posted: October 21, 2013 Filed under: Medical Devices, Professionnal guest | Tags: arrhythmias, atrio-ventricular block, AVB, cardiac, cardiac conduction disturbances, cardiac death, cardio, cardiomyopathy, CCD, HCM, hypertrophic, Hypertrophic cardiomyopathy, myocardial, sudden, sudden cardiac death, tachy-arrhythmias, ventricular Leave a commentCardiac conduction disturbances: the other side of sudden cardiac death in hypertrophic cardiomyopathy.
Hypertrophic cardiomyopathy (HCM) is a myocardial disease characterized by asymmetrical hypertrophy of the heart and disarray of myocardial cells, with an unstable electrical substrate potentially causing lethal ventricular tachy-arrhythmias 1.
Sudden cardiac death is the worst feared complication of hypertrophic cardiomyopathy (HCM), and is known to be mostly related to ventricular arrhythmias such as ventricular fibrillation and ventricular tachycardia 1. However, arrhythmic sudden cardiac death can also be related to cardiac conduction disturbances (CCD), including complete atrio-ventricular block (AVB), possibly leading to prolonged asystolia. The only available treatment for such CCD, is the pacemaker (PM).
Third degree atrio-ventricular block.
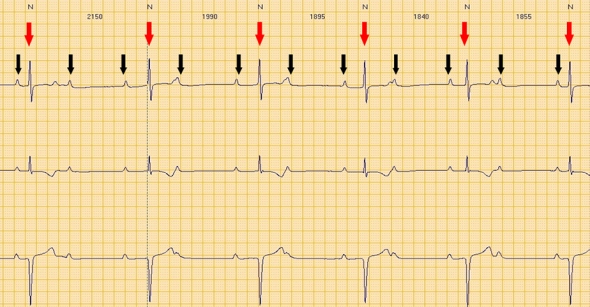
Red arrows: QRS (ventricular electrical activity). Black arrows: P waves (atrial electrical activity).
Atrial and ventricular activity is completely dissociated.
Second degree advanced atrio-ventricular block.
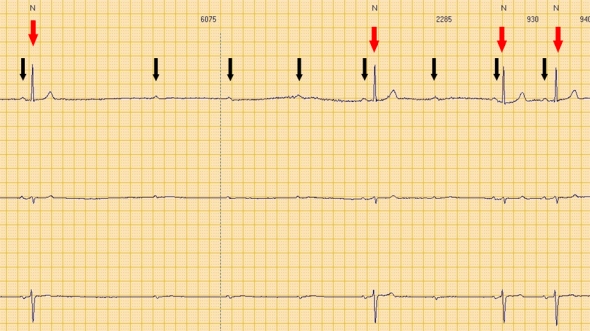
Red arrows: QRS (ventricular electrical activity). Black arrows: P waves (atrial electrical activity).
Consecutive P waves care not followed by QRS, leading to a transient asystolic pause.
Although CCD are commonly seen in association with amyloidosis and glycogen storage disorders, which may themselves be associated with left ventricular hypertrophy 1, little is known about the prevalence of primitive CCD in HCM.
Primitive CCD
Case reports.
A few case reports of HCM patients with complete primitive (i.e. not secondary to surgicalor interventional procedures) AVB have been reported.
Paroxysmal complete AVB has been described in three HCM patients, with prolonged QRS duration at ECG, suffering from recurrent attacks of syncope and cardiopulmonary arrest 2. Symptoms were likely related to the AVB itself.
A case of a north Indian family including nine HCM individuals, among whom 5 underwent PM implantation due to advanced AVB at an age of 39-55 years, has also been described 3. Two sudden cardiac deaths (25 and 51 years of age respectively) were also reported in the same family. The absence of CCD in all the HCM-affected members of the family (therefore sharing the same genetic mutation, likely associated with CCD), may be explained by the phenomenon of incomplete “penetrance” (i.e. the proportion of individuals with the mutation who exhibit clinical symptoms), which in turn is influenced by several factors, including age and gender.
Clinical studies
Prospective studies investigating the prevalence of CCD in HCM requiring permanent PM implantation are lacking. In a retrospective analysis of a small cohort of HCM patients (27 patients; 12 non obstructive) with an implanted PM, a high rate of AVB was observed4. Indications for PM included: spontaneous or induced AVB in 54% of cases (with spontaneous total AVB in over 15% of cases) and support for drug-induced bradycardia in 8% of cases 4.
A larger retrospective study of 451 HCM-patients (44% with previous syncope), also reported a high prevalence of CCD. Overall, PM was implanted in 11% of patients, either due to sinus node dysfunction or to AVB. Interestingly, in 18% of cases, at least one other family member was a PM recipient. One single mutation (E101K in the cardiac actin gene) was identified in 3 index cases (PM recipients). The authors concluded that CCD may form part of the phenotype expression of HCM and may have a familial component 5.
Iatrogenic CCD
Obstruction of the LV outflow tract in HCM, due to septal hypertrophy, is dynamic and varies with loading conditions and contractility of the left ventricle. Interventional procedures for reduction of the left ventricular outflow pressure gradient, such as septal alcohol ablation (SA) or surgical septal myectomy (SM) can be complicated by a not negligible rate of CCDs.
Septal ablation
Trans-catheter alcohol embolization of selected branches of the coronary arteries, aims at generating a circumscribed septal infarction of the left ventricle, in an attempt to reduce the left ventricular outflow tract pressure gradient due to loss of myocardial contraction. The iatrogenic septal infarction usually extends between the anterior and inferior free walls of the left ventricle, in an area commonly containing the right bundle branch (RBB) of the conduction system. Septal Ablation is therefore often complicated by RBB block occurrence 6. AVB has also been described in up to 62% of cases of SA, but is usually transient and characterized by a spontaneous regression within 24 hours. The frequency of CCDs following SA which require permanent PM implantation has ranged between 10% and 33% across studies 6.
Subacute AVB have been also been described, occurring up to 8 days after SA 7. Predictors of subacute AVB post-ASA include: advanced age, prolonged QRS duration before or after SA, new intra-ventricular conduction disturbances or first-degree AV block after SA 7.
Septal myectomy
Septal myectomy entails surgical removal of sub-endocardial tissue in the anterior inter-ventricular septum in an attempt to reduce ventricular contractility and left ventricular outflow tract gradient. Since the left bundle branch fibers of the cardiac conduction system are adjacent to the anterior inter-ventricular septum, patients undergoing SM are at increased risk of developing left bundle branch block. Interestingly, CCDs have been shown to be significantly lower following SM as compared with SA 6,8. The risk of complete AVB is approximately 2% with SM (higher in patients with preexisting RBB block 1 .
Considerations
Current guidelines underline the importance of PM implantation when an advanced CCD occurs after SA or SM 1. However, in planning a PM implantation due to AVB in a HCM-patient, a few considerations must be taken into account, including:
- eventual benefits expected from pacing of the right ventricular apex, in terms of reduction of the left ventricular outflow tract gradient and consequent symptoms reduction1;
- the individual risk of tachy-arrhythmias eventually precipitating sudden cardiac death, with possible indication for cardioverter defibrillator implant.
Nowadays, the availability of sophisticated high-quality PM, such as those provided by NayaMed, allows safe and efficient protection from CCD and associated risk of sudden cardiac death in HCM individuals.
Dr. Annamaria Martino, Dr. Lucia De Luca and Prof. Leonardo Calò
Cardiology Department, Policlinic Casilino, ASL RMB, Rome, Italy.
References
- Gersh J. et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011; 58:e212-260
- Doven O et al. Abnormal His- Purkinje system conduction leading to complete atrioventricular block in patients with hypertrophic cardiomyopathy: a report of 3 cases. Jpn Heart J. 2004;45:347-52.
- Bahl A, Nahar Saikia U, Talwar KK. Familial conduction system disease associated with hypertrophic cardiomyopathy. International Journal of Cardiology 2008; 125: e44–e47
- Alves Silva LA et al. Cardiac Pacing in Hypertrophic Cardiomyopathy. A Cohort with 24 Years of Follow-Up. Arq Bras Cardiol 2008;91:250-256
- Barriales-Villa R et al. Severe cardiac conduction disturbances and Pacemaker implantation in patients with hypertrophic cardiomyopathy. Rev Esp Cardiol. 2010;63:985-8
- Agarwal S et al. Updated meta-analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J Am Coll Cardiol 2010;55:823–34
- Lawrenz T et al. Predictors of complete heart block after transcoronary ablation of septal hypertrophy: results of a prospective electrophysiological investigation in 172 patients with hypertrophic obstructive cardiomyopathy. Am Coll Cardiol. 2007;49:2356-63.
- Talreja DR et al. Alcohol septal ablation versus surgical septal myectomy: comparison of effects on atrioventricular conduction tissue. J Am Coll Cardiol.2004;44:2329-32.
NayaMed products in a few words
Posted: August 20, 2012 Filed under: Medical Devices | Tags: bradycardia, cardiac, cardiac electrophysiology, cardiac techniques, cardiology, conventional devices, defibrillator, eHealthcare, Health 2.0, ICD, Medical Devices, pacemaker, pacemakers, St Jude Medical, technical advisor Leave a commentAs a new appearance on the CRDM Market, customers often ask: “Who are you NayaMed?”, “What are your products and why should we chose you for our patients?”
Well, mostly because we offer a simple online platform with a permanent access to your products, giving full transparency and allowing permanent access to ordering, digital inventory, stock management access and technical support.
Also, because as a clinician, you get the product you need when you need. A product that has been designed and engineered to perfectly match the NayaMed philosophy – Smart and Simple.
We wanted the clinicians to be comfortable when using our devices, so we asked our Technical Advisors to present the NayaMed products in just a few minutes.
Mary Soranno and Marina Mancusi, our technical advisors for the UK and the Italian market did a wonderful job and we are inviting you to see a very brief, but complete presentation of our products in English or Italian.
English Version:
Italian Version:
NayaMed devices made simple. Watch it now!
Posted: August 13, 2012 Filed under: Medical Devices, Uncategorized | Tags: bradycardia, cardiac, cardiac electrophysiology, cardiac techniques, cardiology, conventional devices, defibrillator, eHealthcare, Health 2.0, healthcare applications, heart failure, ICD, Medical Devices, pacemaker, pacemakers, video Leave a commentMeet Mary Soranno, our technical advisor for the UK market presenting all the features of the NayaMed pacemakers & defibrillators. (Better than a long blog post or brochure!).
A new tool to help the patients with moderate to severe Heart Failure
Posted: December 8, 2011 Filed under: Medical Devices | Tags: bradycardia, cardiac, cardiology, heart failure, Medical Devices, pacemakers Leave a commentRecentl y, an Australian company has developed a new heart assisting device designed to help patients suffering of Heart Failure and with mild to moderate symptoms (NYHA II – IV, Stage C).
y, an Australian company has developed a new heart assisting device designed to help patients suffering of Heart Failure and with mild to moderate symptoms (NYHA II – IV, Stage C).
The system represents a balloon that is surrounding the aorta and it inflates and deflates in the same rhythm with the heart contraction. The idea is a new tentative in addressing the heart failure patients that despite optimal drug and device treatment, they still have a poor heart function and significant HF symptoms (shortens of breath, tiredness with moderate activity).
What is interesting is the device tries to improve the cardiac output and also 2 important hemodynamic factors; decrease afterload and recovering a better contractility by a better perfusion of the coronaries. The contractility is an indirect improvement but we could assume that a better perfused myocardium is also contracting better.
In a normal person, the coronary arteries are perfused during diastole. A heart failure patient usually has a long contraction time of the myocardium during the heart cycle which leaves less time for the diastole and therefore less time to perfuse the coronaries.
The apparatus is currently studied in some hospitals in the United States under the FDA guidelines. It consists of a cuff that wraps around the ascending aorta and is connected to a pump by a tube that passes through the skin. This pump is external to the body and it moves air in and out of the cuff. A pacemaker lead is fixated to the heart to detect the appropriate timing when the balloon has to be inflated or deflated. When this one inflates it helps push the blood to the body and to the coronaries. During deflation, the workload or pumping required by the left heart is reduced.
Another notable aspect is that although the device needs heart surgery to be implanted it doesn’t come in direct contact with the blood flow and there is no major incision on the heart except the placement of the pacemaker lead which is minimal.
The constraint that we can think of this tool is that, being external; it has to be permanently carried by the patient in order for this one to take advantage of its benefits. For some activities, like showering the device can be disconnected but not more than 15 minutes, as the manufacturer recommends.
The current clinical results are promising; significant improvements in NYHA Class reduction, Quality of Life, and Left Ventricular Ejection Fraction. This makes the C-Pulse® a possible new treatment for the Heart Failure patients.
We think about the cardiac pacemakers implanted in the fifties that that were initially external and now are implantable and slightly larger than a coin. Maybe in the future this entire new heart assisting system could be minimized and placed completely under the skin.
References:
http://www.sunshineheart.com consulted on the 4th of December 18:00 GMT+1
http://medgadget.com/2011/11/sunshine-hearts-c-pulse-cardiac-pump-proves-itself-in-clinical-trial.html consulted on the 4th of December 18:00 GMT+1
http://www.marketwatch.com/story/sunshine-heart-announces-results-of-its-c-pulser-feasibility-trial-at-the-transcatheter-cardiovascular-therapeutics-conference-2011-11-07 consulted on the 4th of December 18:00 GMT+1
Alexandru Trif
Product Marketing Manager